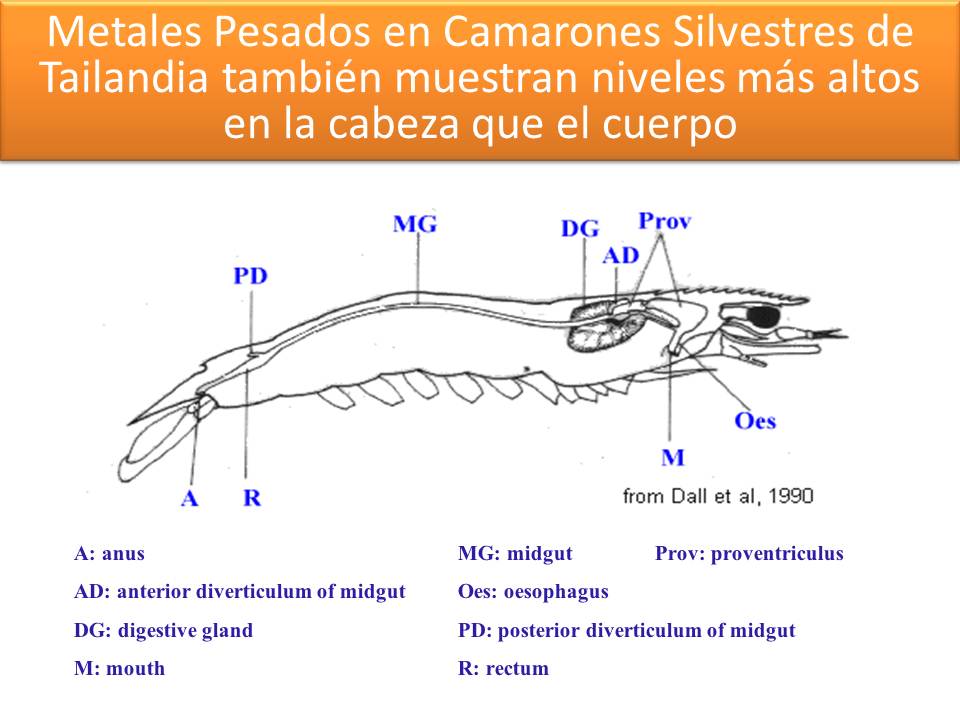
METALES PESADOS EN CAMARONES - POTENCIALES DISRUPTORES ENDOCRINOS A BAJOS NIVELES DE EXPOSICIÓN
Randi Henry1, Acacia Alcivar- Warren1,2*, Carlos Mejia2, Fernando Aveiga2, Miriam Alc2
1Cummings School of Veterinary Medicine at Tufts University, North Grafton, MA USA; 2IMSEGI (International Marine Shrimp Environmental Genomics Initiative) and FUCOBI (Fundacion para la Conservacion de Biodiversidad Acuatica y Terrestre), Guayaquil, Ecuador; [email protected]
Se han publicado numerosos ejemplos documentados de los efectos adversos de los disruptores endocrinos (EDC) en invertebrados, peces, vida silvestre, animales domésticos y humanos.1 Varias sustancias químicas antropogénicas pueden alterar los sistemas hormonales, pero las vías y los objetivos de la alteración endocrina se extienden más allá de los tradicionales. sistemas reproductivos y de desarrollo mediados por receptores de estrógenos / andrógenos / tiroides. Hay nuevas preocupaciones sobre el papel potencial de los EDC en las tendencias crecientes en la pubertad temprana en las niñas, la obesidad y la diabetes tipo II en los EE. UU. Y otras poblaciones, incluidas las alteraciones inducidas por mezclas de sustancias químicas (productos farmacéuticos potentes para humanos y veterinarios, productos para el cuidado personal, nutracéuticos, fitoesteroles) .1 En los crustáceos, el efecto de los EDC, como los contaminantes orgánicos y algunos pesticidas, es inhibir la muda al interferir con la ecdisona en los tejidos diana.2 El cadmio, un metal pesado (Cd), un disruptor endocrino humano, inhibe parte de la secreción de ecdisona en crustáceos. Los xenoestrógenos, juvenoides y ecdisteroides, han producido un desarrollo anormal de los caracteres sexuales secundarios masculinos y femeninos y alteración de la proporción de sexos. El Cd y el cobre (Cu) interfieren con las hormonas que estimulan la reproducción, como el metil farnesoato, así como con la secreción de la hormona inhibidora de las gónadas, por lo que afectan, por ejemplo, el crecimiento ovárico2. Algunos metales pesados producen hiperglucemia en los crustáceos durante breves períodos de exposición; mientras que se observó una respuesta hipoglucémica después de exposiciones más prolongadas, debido a la inhibición de la secreción de la hormona hiperglucémica de los crustáceos.2 En los camarones peneidos, hay poca información disponible sobre el papel de los EDC en el crecimiento, reproducción e inmunidad. Faltan datos para informar a las agencias reguladoras sobre la necesidad de detectar y probar los EDC en la harina de pescado y camarón. La información de referencia sobre metales pesados, PCB y PAH se está recopilando de camarones silvestres y cultivados a través de nuestro proyecto IMSEGI. Se encontraron niveles bajos de Cd en Litopenaeus vannamei silvestres y cultivados de Ecuador y EE. UU. Y Penaeus monodon de Filipinas.3 Otros informes mostraron valores de Cd que iban de 0 a 2.27 ppm en tejidos en diversas etapas del desarrollo del camarón3, algunos de los cuales estaban por encima de la seguridad recomendada. límites de 0,50 mg / kg (ppm) establecidos por la UE4 y la FAO5 y representan un riesgo para la salud pública y animal. Las CL50 de Cd (48 h-30 días) variaron de 0,124 a 5,500 ppm. En esta revisión, los niveles de 15 metales en reproductores silvestres de L. vannamei de tres sitios de Ecuador y uno de El Salvador (Tabla 1) se utilizarán como un estudio de caso para resaltar que las acciones fisiológicas de los EDC se encuentran en concentraciones por debajo de 1 ppm. Hubo diferencias (P <0.05) entre los cuatro sitios para 10 de los 15 metales (Ag, Al, Cd, K, Hg, Pb, Mn, Ni, Se, V). Algunos metales tienen el potencial, en niveles muy bajos, de afectar no solo los sistemas de respuesta inmunológica y reproductiva, sino también la diversidad genética y la aptitud. Se necesita investigación para determinar el efecto de la EDC de los metales y los COP sobre el camarón y la salud humana; la información debe contribuir no solo a la conservación de la biodiversidad del camarón sino también al desarrollo de una industria acuícola sostenible.
1Hotchkiss et al . 2008. Fifteen years after "Wingspread". Toxicol Sci. 105:235-59. 2Rodriguez et al. 2007. Endocrine disruptors in crustaceans. Comp Biochem Physiol A Mol Integr Physiol 146:661-71. 3Keating et al. 2007. Histological findings, cadmium bioaccumulation, in shrimp postlarvae J Shellfish Res 26 (4):1225-1237. 4. 5Report of the Codex Committee on Food Additives and Contaminants. Draft Guideline level for Cadmium in Food
Fuente: https://www.was.org/WASMeetings/meetings/ShowAbstract.aspx?Id=17588
1Hotchkiss et al . 2008. Fifteen years after "Wingspread". Toxicol Sci. 105:235-59. 2Rodriguez et al. 2007. Endocrine disruptors in crustaceans. Comp Biochem Physiol A Mol Integr Physiol 146:661-71. 3Keating et al. 2007. Histological findings, cadmium bioaccumulation, in shrimp postlarvae J Shellfish Res 26 (4):1225-1237. 4. 5Report of the Codex Committee on Food Additives and Contaminants. Draft Guideline level for Cadmium in Food
Fuente: https://www.was.org/WASMeetings/meetings/ShowAbstract.aspx?Id=17588
TRAZAS DE METALES EN LITOPENAEUS VANNAMEI BROODSTOCK DE ECUADOR Y EL SALVADOR
Acacia Alcivar-Warren*, Keith Astrofsky, Jhonny Alcivar, Randi Henry and Dawn M. Meehan
Department of Environmental and Population Health, Tufts University School of Veterinary Medicine, North Grafton, MA 01536
Department of Environmental and Population Health, Tufts University School of Veterinary Medicine, North Grafton, MA 01536
Las poblaciones naturales de camarón L. vannamei están amenazadas por la pérdida de hábitat, contaminantes y enfermedades. Estas presiones ambientales pueden estar afectando la diversidad genética y las características de resistencia a enfermedades de L. vannamei y otras especies marinas. Para desarrollar una industria de acuicultura de camarón sostenible y proteger las poblaciones de camarón silvestre y su hábitat, se necesita información de referencia sobre la presencia de contaminantes, la prevalencia de enfermedades y la diversidad gentil. Trabajando hacia este objetivo, hemos comenzado a desarrollar una base de datos de contaminantes (metales pesados, PCB, HAP) presentes en L. vannamei y P. monodon1. El objetivo de este estudio fue examinar las concentraciones de trazas de 15 metales en reproductores de L. vannamei.
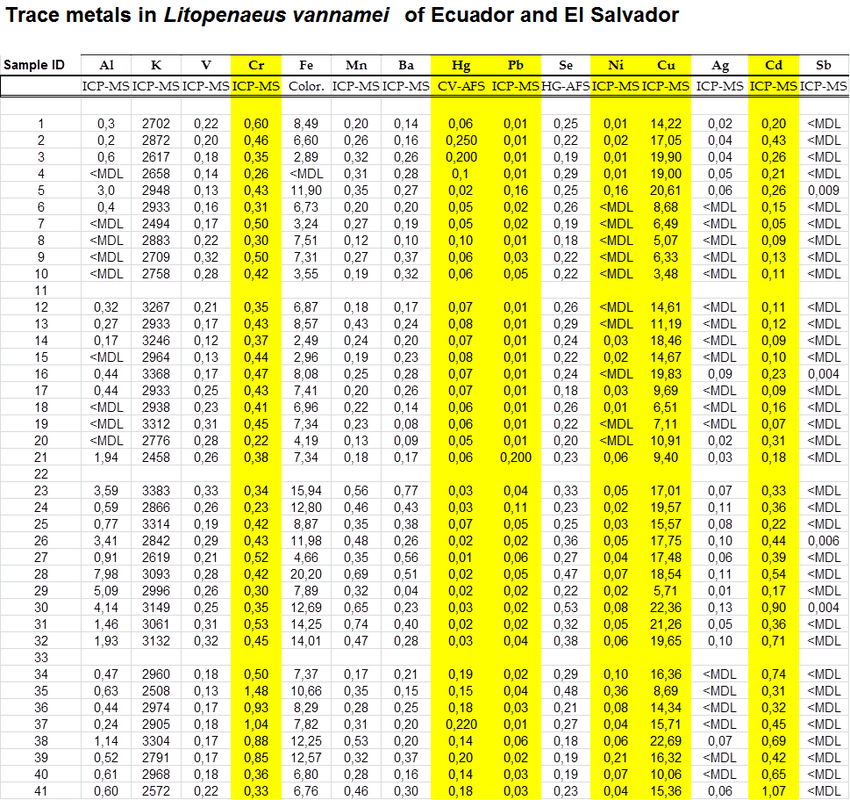
Los reproductores de L. vannamei de Ecuador y El Salvador se mantuvieron en condiciones ambientales similares en un estanque de cultivo con fines de reproducción. Se analizaron diez muestras de cada una de las tres regiones de Ecuador (provincias de Esmeraldas, Manabí y Guayas) y de El Salvador (n = 8) para: aluminio (Al), antimonio (Sb), bario (Ba), cadmio (Cd), cromo (Cr), cobre (Cu), hierro (Fe), plomo (Pb), manganeso (Mn), mercurio (Hg), níquel (Ni), potasio (K), selenio (Se), plata (Ag) y vanadio (V).
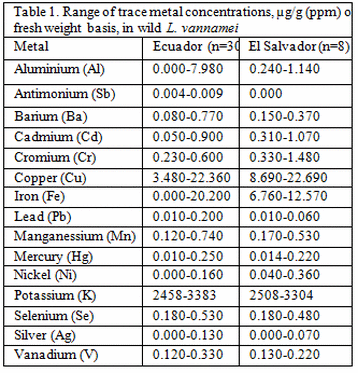
Los 15 metales estaban presentes en L. vannamei adultos. El rango de concentraciones de trazas, µg / g (ppm) sobre la base del peso fresco, varió entre las muestras dentro y entre los sitios. Las concentraciones fueron las siguientes: Al (0.2 a 8.0), Sb (0.004-0.009), Ba (0.04-0.77), Cd (0.046-1.066), Cr (0.22-1.48), Cu (3.48-22.69), Fe ( 2.0-20.00), Pb (0.006-0.195), Mn (0.12-0.74), Hg (0.014-0.250), Ni (0.007-0.357), K (2458-3383), Se (0.18-0.53), Ag (0.014 -0,128) y V (0,12-0,33). Para cada metal, se calculó la concentración media y se usó para clasificar las regiones de mayor a menor. El análisis de los 15 metales mostró que el camarón de Guayas ocupó el primer lugar en el número de metales con concentraciones más altas (10 de 15), seguido por El Salvador (4 de 15) y Esmeraldas (1 de 15). Los reproductores de Manabí se clasificaron como los más bajos (9 de 15) en concentraciones de metales traza. El camarón de El Salvador tuvo las concentraciones de trazas más altas de cuatro metales (Cd, Hg, Cr, Ni) de gran interés para la salud.
Algunas de las concentraciones de metales pesados que se informan aquí tienen el potencial de afectar no solo a los sistemas de respuesta inmunitaria y reproductiva, sino también a la diversidad genética de L. vannamei silvestre. Se ha iniciado un sistema de seguimiento basado en el estado del hábitat, la estructura genética de la población, la prevalencia de enfermedades y la carga contaminante en los camarones silvestres. Esta información debería ser útil para los administradores tanto de los recursos naturales de camarón como de los programas de cría de camarones.
1Alcivar-Warren et al. 2000. Heavy metals, PCBs and PAHs in wild and cultured penaeid shrimp: implications for management of broodstock in breeding programs. In: Proceedings of the Aquaculture and Conservation of Marine Shrimp Biodiversity Symposium (ed. A. Alcivar- Warren), Tufts University School of Veterinary Medicine, December 10, 1998. In press.
Fuente: http://www.aquaculture.ugent.be/Services/newsl/2002/nl148/b1.htm
Los reproductores de L. vannamei de Ecuador y El Salvador se mantuvieron en condiciones ambientales similares en un estanque de cultivo con fines de reproducción. Se analizaron diez muestras de cada una de las tres regiones de Ecuador (provincias de Esmeraldas, Manabí y Guayas) y de El Salvador (n = 8) para: aluminio (Al), antimonio (Sb), bario (Ba), cadmio (Cd), cromo (Cr), cobre (Cu), hierro (Fe), plomo (Pb), manganeso (Mn), mercurio (Hg), níquel (Ni), potasio (K), selenio (Se), plata (Ag) y vanadio (V).
Los 15 metales estaban presentes en L. vannamei adultos. El rango de concentraciones de trazas, µg / g (ppm) sobre la base del peso fresco, varió entre las muestras dentro y entre los sitios. Las concentraciones fueron las siguientes: Al (0.2 a 8.0), Sb (0.004-0.009), Ba (0.04-0.77), Cd (0.046-1.066), Cr (0.22-1.48), Cu (3.48-22.69), Fe ( 2.0-20.00), Pb (0.006-0.195), Mn (0.12-0.74), Hg (0.014-0.250), Ni (0.007-0.357), K (2458-3383), Se (0.18-0.53), Ag (0.014 -0,128) y V (0,12-0,33). Para cada metal, se calculó la concentración media y se usó para clasificar las regiones de mayor a menor. El análisis de los 15 metales mostró que el camarón de Guayas ocupó el primer lugar en el número de metales con concentraciones más altas (10 de 15), seguido por El Salvador (4 de 15) y Esmeraldas (1 de 15). Los reproductores de Manabí se clasificaron como los más bajos (9 de 15) en concentraciones de metales traza. El camarón de El Salvador tuvo las concentraciones de trazas más altas de cuatro metales (Cd, Hg, Cr, Ni) de gran interés para la salud.
Algunas de las concentraciones de metales pesados que se informan aquí tienen el potencial de afectar no solo a los sistemas de respuesta inmunitaria y reproductiva, sino también a la diversidad genética de L. vannamei silvestre. Se ha iniciado un sistema de seguimiento basado en el estado del hábitat, la estructura genética de la población, la prevalencia de enfermedades y la carga contaminante en los camarones silvestres. Esta información debería ser útil para los administradores tanto de los recursos naturales de camarón como de los programas de cría de camarones.
1Alcivar-Warren et al. 2000. Heavy metals, PCBs and PAHs in wild and cultured penaeid shrimp: implications for management of broodstock in breeding programs. In: Proceedings of the Aquaculture and Conservation of Marine Shrimp Biodiversity Symposium (ed. A. Alcivar- Warren), Tufts University School of Veterinary Medicine, December 10, 1998. In press.
Fuente: http://www.aquaculture.ugent.be/Services/newsl/2002/nl148/b1.htm